2. The lipid bilayer is
fluid-like, permitting movement of imbedded molecules (lipids, proteins) sideways within the membrane.
- A classic experiment by Frye and Edidin showed that imbedded protein molecules could move from place to place on the cell membrane. (cell fusion and labeling with fluorescent dyes). More recently, Edidin used Near-field Scanning Optical Microscopy at Johns Hopkins, where he did the original membrane fluidity studies more than 30 years ago.
- Membrane fludity is affected by the placement and number of double bonds in the fatty acid tails (which cause kinks and therefore spaces between the adjacent lipids) and the amount of cholesterol, which fills in these spaces and stabilizes the membrane structure.
- Some fish adjust the proportion of different lipids as they migrate from waters of one temperature to another. Bacteria grown in different temperature regimes also show differences in the lipid composition of cell membranes.
3. The lipid membrane is asymmetrical
- Notice that we have glycolipids pointing to the outside of the cell. Glycolipids have a carbohydrate chain as the "head" group and have functions as identifiers of cell type.(A, B, O blood groups are the result of different glycolipids on the surface of red blood cells.) And we have lipids like phosphatidylinositol on the inside of the cell membrane. Phosophatidylinositol is one of the lipids which are broken down and the pieces used as internal cell signals. (Diagram of asymmetric distribution of lipids), (Distribution of lipids)
- Glycolipids and glycoproteins play a major role in signaling cell characteristics to the outside world. For example, A, B, O blood groups result from having different carbohydrate chains on the cell surface glycolipids and glycoproteins of red blood cells and other types of cells. Everyone has glycolipids and glycoproteins which have a particular type of carbohydrate chain which signals type O. People with type A also have glycolipids and glycoproteins which have an carbohydrate called N-Acetylgalactosamine added to the basic type O carbohydrate chain. People with type B have an added galactose. Those with type AB have some glycolipids and glycoproteins with N-Acetylgalactosamine added and other glycolipids and glycoproteins with galactose added. (fig. 10-20, Lodish)
4. Membranes have transmembrane and surface proteins.
Most transmembrane proteins have alpha-helicies spanning the membrane. These helices are different from the alpha-helix of keratin, in that transmembrane helices have hydrophobic side chain groups pointing outward towards the lipid core of the membrane. Such membrane proteins may be single-pass (fig. 10-15, Lodish), as in glycophorin A, or multipass (fig. 10-16, Lodish), as in bacteriorhodopsin.
Aquaporins are transmembrane proteins that allow diffusion of water through cell membranes. Aquaporins also have alpha-helical segments spanning membranes. (fig. 11-8, Lodish)
Porins are transmembrane proteins that allow diffusion of small molecules, including some ions, sugars, and amino acids. Most porins have a membrane-spanning barrel that consists of beta-sheet, not alpha-helix! Amino acids tend to alternate between hydrophobic and hydrophilic, with the hydrophobin chains pointing outward to the membrane interior and the hydrophilic side chains pointing towards the water filled interior of the pore.(fig. 10-18, Lodish).
Some surface membrane proteins are covalently bonded to a hydrocarbon chain that sits in the membrane. (fig. 10-19, Lodish).
Please be especially careful to read pages 421-426 in Lodish. You will be examined on the details of this material.

 All text and images, not attributed to others, including course examinations and sample questions, are Copyright, 2008, Thomas J. Herbert and may not be used for any commercial purpose without the express written permission of Thomas J. Herbert.
All text and images, not attributed to others, including course examinations and sample questions, are Copyright, 2008, Thomas J. Herbert and may not be used for any commercial purpose without the express written permission of Thomas J. Herbert.


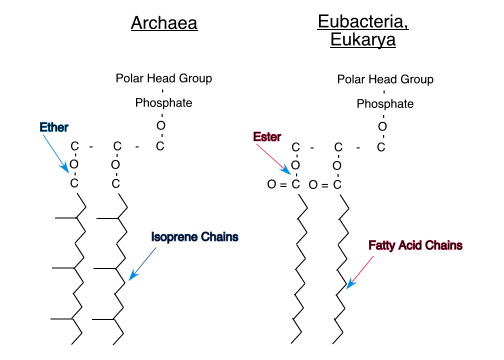

 All text and images, not attributed to others, including course examinations and sample questions, are Copyright, 2008, Thomas J. Herbert and may not be used for any commercial purpose without the express written permission of Thomas J. Herbert.
All text and images, not attributed to others, including course examinations and sample questions, are Copyright, 2008, Thomas J. Herbert and may not be used for any commercial purpose without the express written permission of Thomas J. Herbert.