2. Biochemical reactions are aided by enzymes.
- Lysozyme is a good example of catalysis by a simple enzyme.
("Cartoon of lysozyme working") and
("Mechanism of lysozyme reaction")

- Michaelis-Menten kinetics describes many enzymes which follow a simple reaction scheme of E + S <-> ES -> E + P, where E is enzyme, S is substrate, ES is the bound enzyme-substrate complex, and P is the product.(fig. 3-23, Lodish) (fig. 3-22a, Lodish) (fig. 3-22b, Lodish).
("Reaction rate vs. substrate concentration")
-
With this reaction scheme and three additional assumptions:
- 1) The rate constant of the reaction, E + P -> EP, is zero,
- 2) S >> E, and
- 3) steady state conditions,
the chemical reaction equations can be
solved to give the fundamental equation of Michaelis-Menten kinetics:
where V is the velocity of the reaction - the rate of product formation
in mMoles/liter-second. The constants Vmax and Km characterize the
particular reaction - For example the value of Km depends upon the
values of the rate constants for the reaction. Note that Vmax is the
maximum velocity - which is approached as substrate concentration
approaches infinity.
-
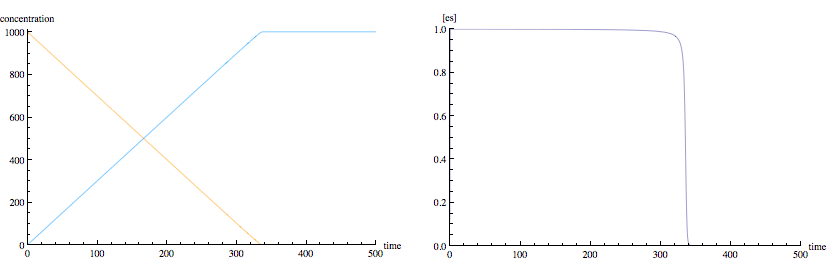
I've simulated a laboratory experiment with an enzyme catalyzed reaction where conditions 1) and 2) (above) are met but without the assumption of steady state kinetics. Notice that the particular starting concentrations and rate constants used in my example (typical of real reactions) results in very close to steady-state conditions for the enzyme-substrate complex. (The left panel shows substrate concentration in orange and product concentration in blue. The right panel shows enzyme concentration.)
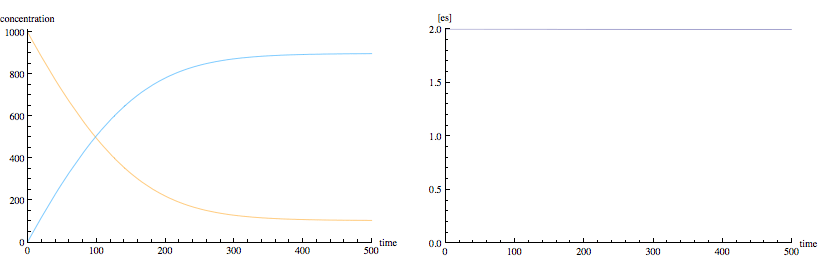
The following is the same enzyme kinetics scheme modified to permit product to combine with enzyme to go backwards to form enzyme-substrate complex. Notice that we now have typical equilibrium kinetics, with exponential rise and fall of concentrations to non-zero equilibrium levels. However, real enzymes usually don't permit product to combine with the enzyme.
- Competitive inhibitors increase the apparent KM while Vmax is unchanged. Non-competitive inhibitors decrease the apparent Vmax while KM is unchanged. Look at this
picture to see how the V vs. S plot changes when inhibitors are present.
- A
Lineweaver-Burke plot can help determine if an enzyme obeys Michaelis-Menten kinetics. Lineweaver-Burke replotted Michaelis-Menten data by plotting 1/V vs. 1/S instead of V vs. S. When data points are replotted on the 1/V vs. 1/S plot, they lie on a straight line IF the data obey Michaelis-Menten kinetics. Your instructor will discuss how the following cases are related to the V vs. S plots. This first graph represents Michaelis-Menten kinetics with increasing concentrations of a competitive inhibitor:

and the second plot with increasing concentrations of a non-competitive inhibitor:

- (Obtain the algebraic expressions for the slope and intercept of an Eadie-Hofstee plot. There will be a question about this on the first exam. Hint - either rearrange the Michaelis-Menten equation to derive the answers yourself or search the web to find the answers.)
- Some enzymes are multisubunit. These "allosteric" enzymes have a sigmoid (S-shaped) relationship between reaction velocity and substrate concentration.

One of the relationships above is for an enzyme plus substrate alone, one is for an enzyme acting in presence of an inhibitor, and the remaining curve is for an enzyme acting in the presence of an activator. Do you know which is which? Do you see how a small change in enzyme structure and the resulting small change in the V vs. S relationship can cause a large change in reaction velocity?)
(Compare Michaelis-Menten and allosteric kinetics to oxygen binding by myoglobin and hemoglobin. Myoglobin has a binding curve identical in shape to that for Michaelis-Menten kinetics but hemoglobin has an sigmoid binding curve, like the V vs S relationship for allosteric enzymes.)
- Aspartate Transcarbamoylase (ATCase) is a multisubunit enzyme that catalyzes a reaction which leads to the synthesis of the pyrimidine ring of C, U, and T nucleotides. CTP, one of these products, is an inhibitor of this enzyme and ATP is an activator.










 All text and images, not attributed to others, including course examinations and sample questions, are Copyright, 2008, Thomas J. Herbert and may not be used for any commercial purpose without the express written permission of Thomas J. Herbert.
All text and images, not attributed to others, including course examinations and sample questions, are Copyright, 2008, Thomas J. Herbert and may not be used for any commercial purpose without the express written permission of Thomas J. Herbert.